NMR Titration. The protonation constants K1 and K2 of a diprotic acid H2A were determined by NMR titration. (Protonation constants, for example,
![]()
are used in this example because they simplify the equilibrium expressions The chemical shift S of a hydrogen near the acidic sites was measured at a number of pH values over the range pH 1 to pH 11. The data are shown in the following Figure (data table and figure are on the CD that accompanies this book).
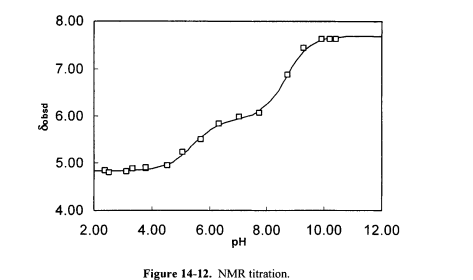
At any pH value there are three acid-base species in solution: H2A, HAand A2-; the observed chemical shift is given by the expression
![]()
where a, is the fraction of the species in the form containing j acidic hydrogens and q is the chemical shift of the species. The a values can be calculated using the expressions below:
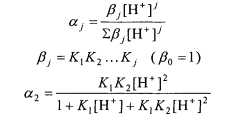
Use the Solver to determine KI, K2, &, 61 and 6;.

