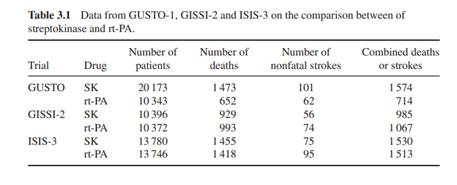
The GUSTO-1 study is a mega-sized RCT comparing two thrombolytics: streptokinase (SK) and rt-PA for the treatment of patients with an acute myocardial infarction. The outcome of the study is 30-day mortality, which is a binary indicator whether the treated patient died after 30 days or not. The study recruited 41 021 acute infarct patients from 15 countries and 1081 hospitals in the period December 1990–February 1993. The basic analysis was reported in The GUSTO Investigators (1993) and found a statistically significant lower 30-day mortality rate for rt-PA compared with SK. Brophy and Joseph (1995) reanalysed the GUSTO-1 trial results from a Bayesian point of view, leaving out the subset of patients who were treated with both rt-PA and SK. As prior information, the data from two previous studies have been used: GISSI-2 and ISIS-3. In Table 3.1, the data from the GUSTO-1 study and the two historical studies are given. The authors compared the results of streptokinase and rt-PA using the difference of the two observed proportions, equal to absolute risk reduction (ar), of death, nonfatal stroke and the combined endpoint of death or nonfatal stroke. Use the asymptotic normality of ar in the calculations below.
1. Determine the normal prior for ar based on the data from (a) the GISSI-2 study, (b) the ISIS-3 study, and (c) the combined data from the GISSI-2 and ISIS-3 studies.
2. Determine the posterior for ar for the GUSTO-1 study based on the above priors and a noninformative normal prior.
3. Determine the posterior belief in the better performance of streptokinase or rt-PA based on the above-derived posterior distributions.
4. Compare the Bayesian analyses to the classical frequentist analyses of comparing the proportions in the two treatment arms. What do you conclude?
5. Illustrate your results graphically.