1. The emf of the following cell Mg(Z) I MgC12-CaC12(1) 1 MgsSi(s), Si(s) was found to be &O = 0.21767 -8.6O7x1V5T, (V) Express the standard free energy of formation of Mg2Si(s) as a function of temperature.
2. The emf of the following cell was measured at a number of different temperatures : Cu20(s), CuO(s) I Zr02(+CaO) I 02(g, air) The results were reported as follows : Calculate the standard free energy of formation of CuO(s) as a hction of temperature. The free energy of formation of Cu20 is given : AG;,cu,o = -168,400+ 71.25T, J/mole.
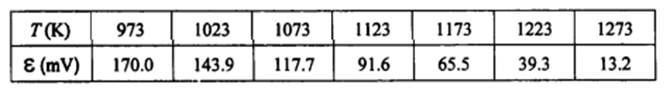
Calculate the standard free energy of formation of CuO(s) as a hction of temperature. The free energy of formation of Cu20 is given : AG;,cu,o = -168,400+ 71.25T, J/mole

