The W-visible spectra of aqueous solutions of CoC12, NiCI2 and CuC12 are shown in Figure 9- 16.
Figure 9-16
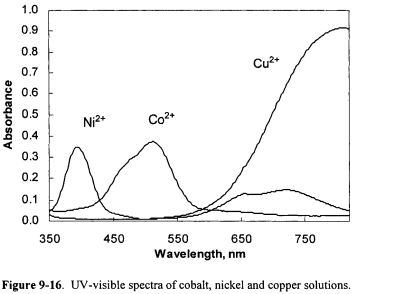
Three wavelengths were chosen at which the absorbance of the three species, Co2+, Ni2+ and Cu2+, differed significantly. The molar absorptivities of the three species at the three wavelengths are shown in Table 9-1.
Table 9-1

A mixture of the three metal ions gave the following absorbance readings at the three wavelengths: 394 nm, 0.845; 510 nm, 0.388; 808 nm, 1.696, when measured using a cell with a 1.00-cm path length. Calculate the concentration of the three metal ions in the mixture, using Beer’s Law: A = ~bc (A = absorbance, E= molar absorptivity, b = cell path length in cm, c = concentration in mol/L).

