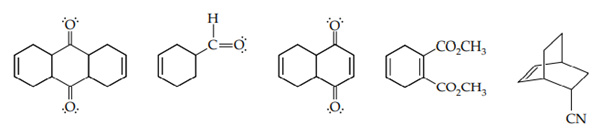
i. What starting materials would you use to prepare each of the following compounds by the Diels–Alder reaction?
ii. Diels–Alder reactions with benzene are rare, and require a very reactive (electron-deficient) dienophile, because benzene is a rather unreactive diene. Two are shown below. Give the structures of the product produced in each reaction.
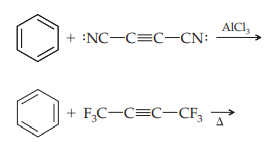
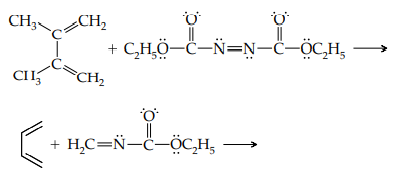
iii. Shown below are two heteroatom compounds that undergo the Diels–Alder reaction. Formulate the product obtained in each reaction.