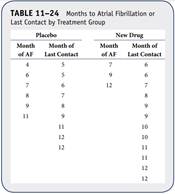
Using the data in Problem 2, compute and interpret the hazard ratio for atrial fibrillation with the new antiarrhythmic drug as compared to placebo.
Problem 2
A clinical trial is run to assess the effectiveness of a new antiarrhythmic drug designed to prevent atrial fibrillation (AF). Thirty participants enroll in the trial and are randomized to receive the new drug or placebo. The primary outcome is AF, and participants are followed for up to 12 months following randomization. The experiences of participants in each arm of the trial are shown in Table 11–24.
a. Estimate the survival functions for each treatment group using the Kaplan–Meier approach.
b. Test whether there is a significant difference in survival between treatment groups using the log-rank test and a 5% level of significance.