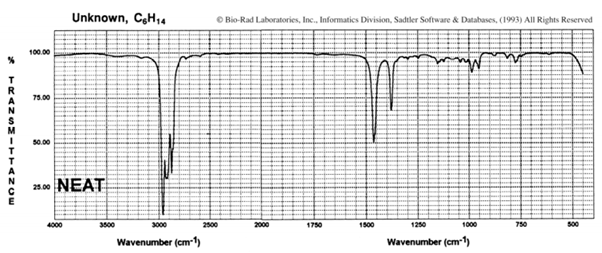
i. Suggest a possible structure for the hydrocarbon C6H14, which has the infrared spectrum shown here: Is there more than one correct structure?
ii. The hydroxylamine I can be oxidized by MnO2 to the amide oxohaemanthidine (II). In dilute solution, the carbonyl absorption band of II occurs at 1702 cm–1 . Explain this observation.
a. 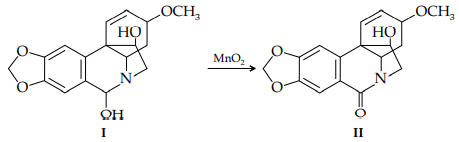
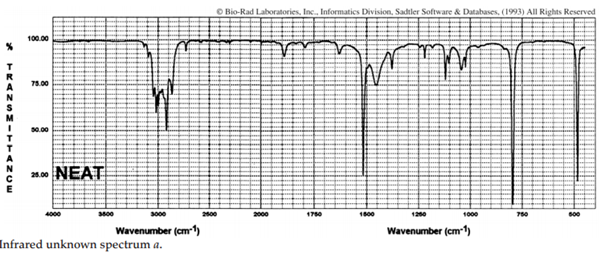
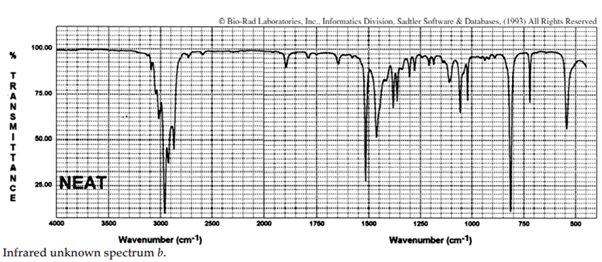
iii. The infrared spectra of the three xylene (dimethylbenzene) isomers, and an additional aromatic hydrocarbon, are given below. Assign the spectra to the isomers and suggest a potential structure for the remaining unknown substance.
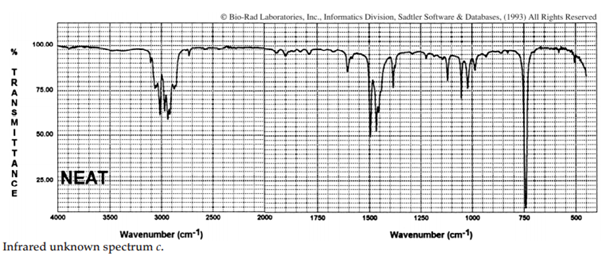
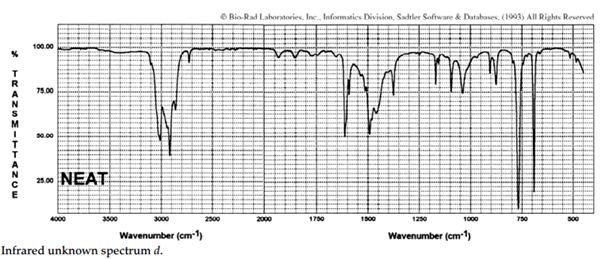
iv. The COH stretching mode of chloroform (CHCl3), which occurs at 3022 cm–1 , is one of the rare exceptions to the 3000-cm–1 rule. What is the rule? Suggest an explanation for this exception.

