1.The melting point of CaTiSiOs is 1,400″C and the heat of fusion at the normal melting point is 123,700J mol-‘. Calculate the heat of fusion at 1,300″C. Cp,sd,d= 177.4 + 23.2xlO”T- 4O.3x1O5T2, J mol-‘K’ Cp,liguid = 279.6 J mol”K”
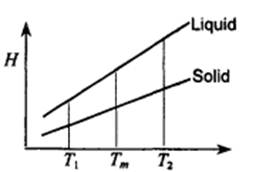
2. Enthalpy changes resulting from temperature change can be represented on an enthalpy-temperature diagram as shown in the figure. Express on the diagram the answers to the following questions:
1) Enthalpy change when solid A melts at T,,,
2) Enthalpy change when liquid A is super cooled
3) Enthalpy change when solid A is superheated from T,,, to T,, and then solidifies. from T,,, to T, and then melts