i. Explain why the 25% NaOH solution is added to the reaction mixture.
ii. Draw a structure to illustrate the hydrogen bonding that may occur when two polymer molecules of this polyamide are cold – drawn together.
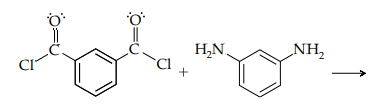
iii. Predict the structure of the polymer that would result in the condensation of the following reactants. These monomers are used to produce the polyamide Nomex, a high-melting material used as an insulator in space shuttles and as the fire-resistant fabric in clothing worn in race cars.
iv. Amides undergo hydrolysis to carboxylic acids on treatment with alkali. Diagram a suitable mechanism for the conversion of benzamide to benzoic acid using sodium hydroxide as the base.

