Determine the flow rates of all components entering the reactor (mol/h) and product stream from…
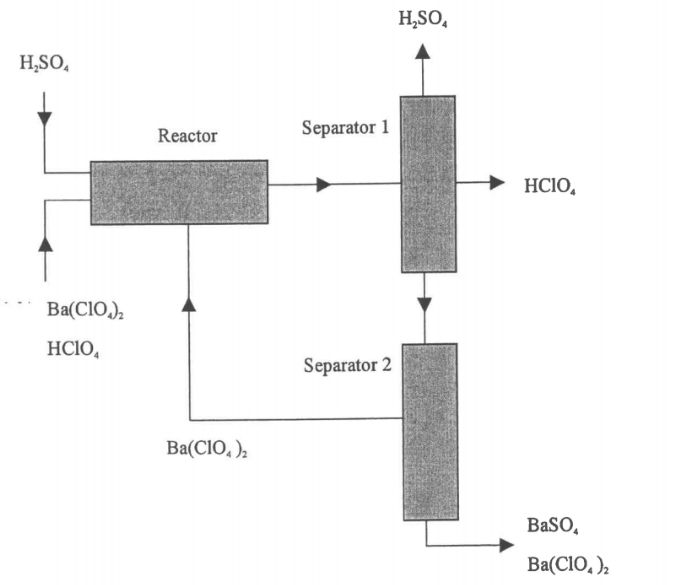 Given :MW (H2SO4) =98.08MW(HCLO4) =100.46MW (Ba(CLO4)2) =336.25MW (BaSO4) =233.41The reaction follows stoichiometry.Ba (CIO4)2 + H2SO4 ? BaSO4 + 2 HCIO4The fed H2SO4 is 25% in excess of stoichiometric amount required for reaction with Ba(CLO4)2.Amount of 100kg/h of stream containing 85% Ba(CLO4)2 and the balance HCLO4 is fed to the reactor.The percentage conversion of Ba(CLO4)2 is 35% per pass.The product of separator 2 contains 3% of Ba(CLO4)2 and the balance BaSO4.
Given :MW (H2SO4) =98.08MW(HCLO4) =100.46MW (Ba(CLO4)2) =336.25MW (BaSO4) =233.41The reaction follows stoichiometry.Ba (CIO4)2 + H2SO4 ? BaSO4 + 2 HCIO4The fed H2SO4 is 25% in excess of stoichiometric amount required for reaction with Ba(CLO4)2.Amount of 100kg/h of stream containing 85% Ba(CLO4)2 and the balance HCLO4 is fed to the reactor.The percentage conversion of Ba(CLO4)2 is 35% per pass.The product of separator 2 contains 3% of Ba(CLO4)2 and the balance BaSO4.
Assume all composition are on weight basis
(i)Redraw and label the flowchart of the process
(ii)Determine the flow rates of all components entering the reactor (mol/h) and product stream from separator 2.
(iii)Determine the flow rates of all components entering separator 1 and all streams leaving separator 1 including the recycle stream.

