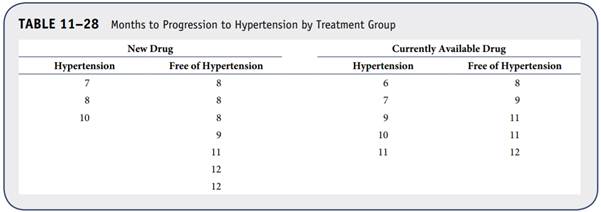
A clinical trial is conducted to evaluate the efficacy of a new drug for prevention of hypertension in patients with prehypertension (defined as systolic blood pressure between 120 mmHg and 139 mmHg or diastolic blood pressure between 80 mmHg and 89 mmHg). A total of 20 patients are randomized to receive the new drug or a currently available drug for treatment of high blood pressure. Participants are followed for up to 12 months, and time to progression to hypertension is measured. The experiences of participants in each arm of the trial are shown in Table 11–28.
a. Estimate the survival functions (time to progression to hypertension) for each treatment group using the Kaplan–Meier approach.
b. Test whether there is a significant difference in time to progression between treatment groups using the log-rank test and a 5% level of significance.
c. Compute and interpret the hazard ratio for time to progression comparing treatment groups.